
icon
-
Story
 Addressing unmet needs for inherited neuromuscular diseases
Addressing unmet needs for inherited neuromuscular diseases -
Press ReleaseSandoz receives European Commission approval for Tyruko® (natalizumab), first and only biosimilar for multiple sclerosis in Europe
-
Story
 In neuroscience, “an inflection point in knowledge and technology”
In neuroscience, “an inflection point in knowledge and technology” -
Press ReleaseNew Novartis extension phase data show nearly 80% of RMS patients treated with Kesimpta® (ofatumumab) had no evidence of disease activity (NEDA-3)
-
Press ReleaseNovartis presents new four-year data on efficacy and safety of Kesimpta® (ofatumumab) in people living with relapsing multiple sclerosis
-
Press ReleaseNew peer-reviewed research shows no increased risk of serious COVID-19 infections in Kesimpta® (ofatumumab)-treated adults with multiple sclerosis
-
Press ReleaseNovartis, Voyager Therapeutics reach license option agreement for next-generation gene therapy vectors for neurological diseases
-
Press ReleaseNovartis announces U.S. Court of Appeals for the Federal Circuit (CAFC) upholds validity of Gilenya® (fingolimod) dosage regimen patent
-
Press ReleaseKesimpta® (ofatumumab) data at ECTRIMS highlights preservation of IgG levels and safety experience over extended exposure (~3.5 years) in people living with relapsing multiple sclerosis
-
Story
 Using Super-Resolution Microscopy To See Neurodegeneration
Using Super-Resolution Microscopy To See Neurodegeneration -
Story
 Neuroscientists target Alzheimer’s “silent stage”
Neuroscientists target Alzheimer’s “silent stage” -
Story
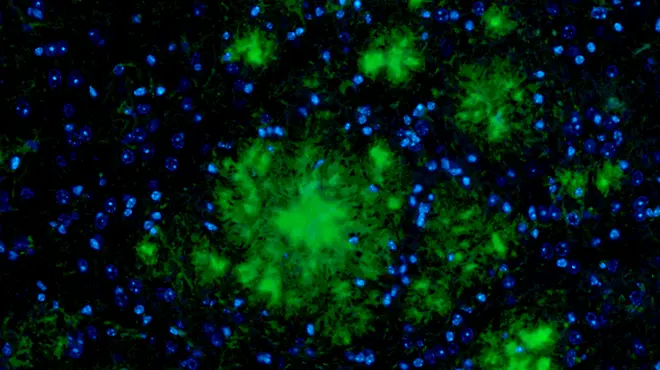 Compound designed to fight Alzheimer’s disease shows promise in the lab
Compound designed to fight Alzheimer’s disease shows promise in the lab